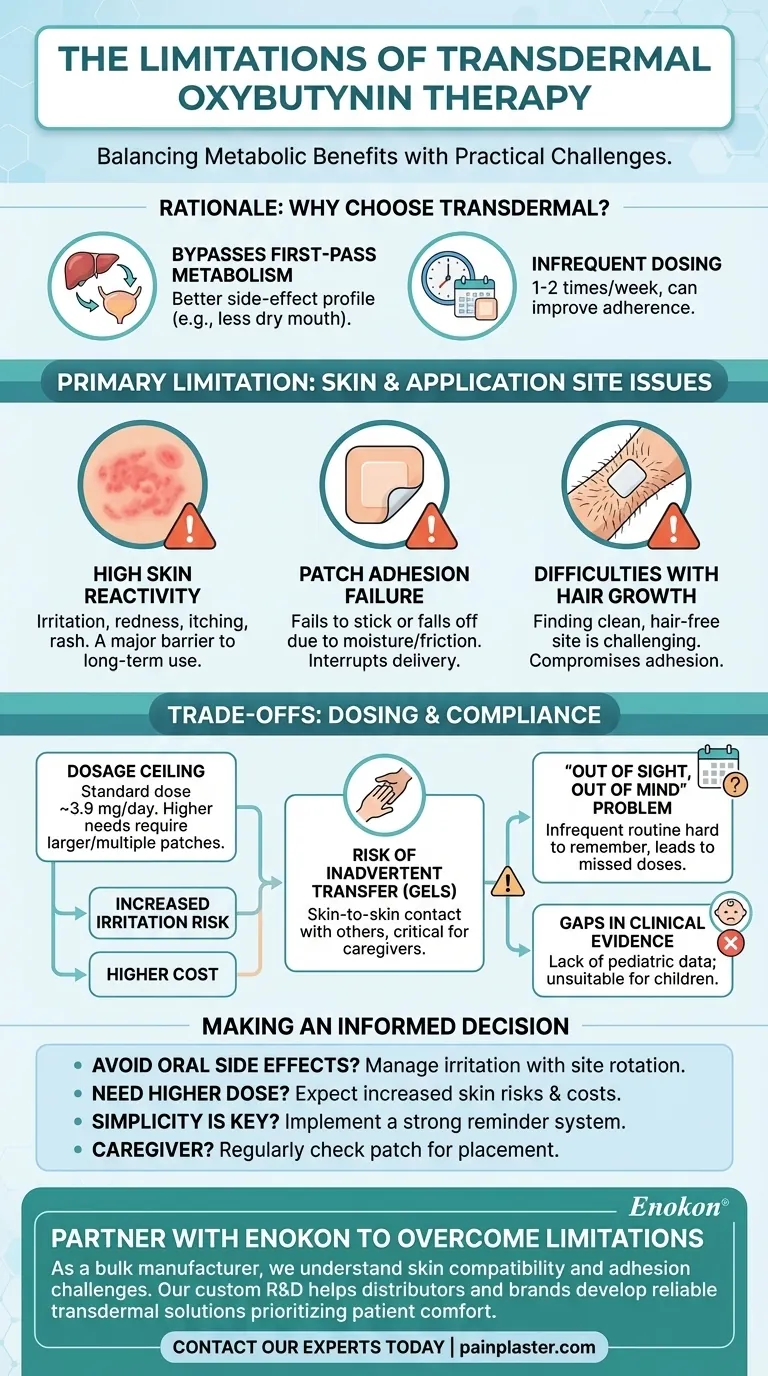
Transdermal oxybutynin therapy offers controlled drug delivery for conditions like overactive bladder but has notable limitations. Key issues include skin reactivity (itching, redness, or rash in up to 16.8% of users), dosage constraints (fixed 3.9 mg/day delivery requiring multiple patches for higher doses), and anatomical challenges for male patients due to body hair. Pediatric use lacks data, and drug interactions with anticholinergics or CNS depressants require caution. While proper skin care and site rotation mitigate some irritation, the system remains unsuitable for hydrophilic drugs or patients needing rapid dose adjustments.

Key Points Explained:
-
Skin Reactivity and Irritation
- Up to 16.8% of users experience pruritus (itching), with 5.6–8.3% reporting erythema (redness) at the application site.
- Mild reactions typically resolve after patch removal, but cumulative irritation may occur without proper site rotation.
- Hair growth in males can complicate adhesion and increase irritation risk, making the Oxybutynin Transdermal Patch less ideal for this group.
-
Dosage Limitations
- The patch delivers a fixed 3.9 mg/day dose via a 39 cm² matrix. Higher doses require multiple patches, escalating costs and skin reaction risks.
- Inflexibility makes it unsuitable for patients needing rapid titration or those with variable dosing needs.
-
Demographic Restrictions
- No established safety or efficacy data for pediatric use.
- Body hair in males may interfere with adhesion, limiting application sites (abdomen, buttock, hip).
-
Drug Interactions
- Concurrent use with anticholinergics (e.g., dry mouth, constipation) or CNS depressants (e.g., drowsiness) requires careful monitoring.
-
General Transdermal System Constraints
- Only small-molecule drugs (like oxybutynin) penetrate skin effectively; hydrophilic medications are incompatible.
- Skin barrier variability (age, hydration) can alter drug absorption unpredictably.
-
Mitigation Strategies
- Patient education on skin care (clean/dry application, site rotation) and topical steroids for irritation can improve adherence.
- Avoiding irritants (e.g., alcohol-based products) near application sites reduces reactivity.
These factors collectively shape clinical decision-making, particularly for patients with sensitive skin, complex dosing needs, or concurrent medications.
Summary Table:
| Limitation | Details | Mitigation Strategies |
|---|---|---|
| Skin Reactivity | Itching, redness, or rash in up to 16.8% of users. | Rotate application sites, use topical steroids. |
| Dosage Constraints | Fixed 3.9 mg/day delivery; higher doses require multiple patches. | Consider alternative therapies for flexible dosing. |
| Demographic Restrictions | No pediatric data; adhesion issues in males due to body hair. | Educate patients on proper skin preparation. |
| Drug Interactions | Risk with anticholinergics or CNS depressants. | Monitor patients closely for adverse effects. |
| General Constraints | Only small-molecule drugs penetrate skin effectively. | Avoid hydrophilic medications. |
Looking for reliable transdermal solutions tailored to your needs? Contact Enokon today to discuss custom R&D and bulk manufacturing of transdermal patches and pain plasters. Benefit from our technical expertise and high-quality production for healthcare and pharmaceutical distributors. Let’s develop solutions that overcome common therapy limitations together!
Guida Visiva
Prodotti correlati
- Cerotto antidolorifico naturale a base di erbe di assenzio
- Cerotti antidolorifici al calore dell'infrarosso lontano Cerotti transdermici
- Cerotti per il sollievo dal calore profondo a infrarossi lontani Cerotti medicati per il sollievo dal dolore
- Cerotto per la tosse e il dolore da asma per adulti e bambini
- Cerotti riscaldanti antidolorifici per i crampi mestruali
Domande frequenti
- Quali condizioni mediche possono essere trattate con i cerotti?Esplora le soluzioni transdermiche versatili
- Quali sono i due tipi di cerotti alla nicotina disponibili e come si differenziano?Scegliere il cerotto giusto per smettere di fumare
- Perché un sistema HPLC è essenziale per gli esperimenti transdermici? Garantire un'analisi precisa della penetrazione dei farmaci
- Come si applicano i cerotti transdermici di estrogeni e progestinici?Guida passo-passo alla terapia ormonale
- Qual è il farmaco discusso per la somministrazione transdermica nei piccoli animali?Fentanil citrato per il trattamento del dolore in veterinaria
- Cosa si deve discutere con un operatore sanitario prima di usare il metilfenidato transdermico?Considerazioni chiave sulla sicurezza e sull'efficacia
- Quali precauzioni si devono prendere quando si usa il cerotto alla scopolamina?Consigli di sicurezza essenziali per un uso efficace
- Quali sono gli usi alternativi della clonidina transdermica oltre al trattamento dell'ipertensione?Esplorare applicazioni versatili

















